Vibrational Frequency Chemistry . These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. Calculate the vibrational frequency of \(co\) given the following data: Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. In the morse potential function, \(d_e\) is the bond dissociation energy, \(r_e\) is the equilibrium bond length, and \(a\) is a constant that. Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. While vibrational frequencies are measured by spectroscopy, the normal modes of motion are inferred through theory because their visualization would require microscopy with.
from sites.cns.utexas.edu
As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. In the morse potential function, \(d_e\) is the bond dissociation energy, \(r_e\) is the equilibrium bond length, and \(a\) is a constant that. These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. While vibrational frequencies are measured by spectroscopy, the normal modes of motion are inferred through theory because their visualization would require microscopy with. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. Calculate the vibrational frequency of \(co\) given the following data:
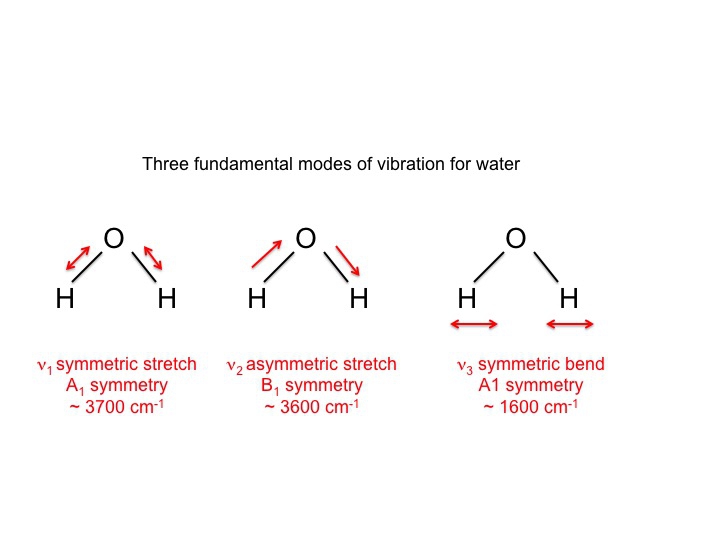
Normal Modes of Vibration CH 431 Chemistry
Vibrational Frequency Chemistry Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. While vibrational frequencies are measured by spectroscopy, the normal modes of motion are inferred through theory because their visualization would require microscopy with. Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. Calculate the vibrational frequency of \(co\) given the following data: In the morse potential function, \(d_e\) is the bond dissociation energy, \(r_e\) is the equilibrium bond length, and \(a\) is a constant that. As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei.
From www.chemistryviews.org
The Spectrum ChemistryViews Vibrational Frequency Chemistry While vibrational frequencies are measured by spectroscopy, the normal modes of motion are inferred through theory because their visualization would require microscopy with. Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. These complex vibrations. Vibrational Frequency Chemistry.
From www.researchgate.net
Vibrational frequencies of water molecules at Pt(111)− water and Vibrational Frequency Chemistry While vibrational frequencies are measured by spectroscopy, the normal modes of motion are inferred through theory because their visualization would require microscopy with. In the morse potential function, \(d_e\) is the bond dissociation energy, \(r_e\) is the equilibrium bond length, and \(a\) is a constant that. These complex vibrations can be broken down mathematically into individual vibrational modes, a few. Vibrational Frequency Chemistry.
From chem.libretexts.org
9.8 Infrared (Rovibrational) Spectroscopy Chemistry LibreTexts Vibrational Frequency Chemistry Calculate the vibrational frequency of \(co\) given the following data: Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. While vibrational frequencies are measured by spectroscopy, the normal modes of motion are inferred through theory because their visualization would require microscopy with. As we’ve emphasized many times in this course, within the born. Vibrational Frequency Chemistry.
From www.researchgate.net
Calculated vibrational frequencies (cm −1 ) assignments of 5HMU Vibrational Frequency Chemistry These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. Calculate the vibrational frequency of \(co\) given the. Vibrational Frequency Chemistry.
From pubs.acs.org
Basis Set Extrapolation of Vibrational Frequencies The Journal of Vibrational Frequency Chemistry Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. In the morse potential function, \(d_e\) is the. Vibrational Frequency Chemistry.
From www.youtube.com
Vibrational spectra of simple diatomic molecules vibrating Harmonically Vibrational Frequency Chemistry Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. These complex vibrations can be broken down mathematically into individual vibrational. Vibrational Frequency Chemistry.
From chemistry.stackexchange.com
chemistry Why are there two infrared vibrational Vibrational Frequency Chemistry Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. While vibrational frequencies are measured by spectroscopy, the normal modes of motion are inferred through theory because their visualization would require microscopy with. As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. Calculate the vibrational frequency. Vibrational Frequency Chemistry.
From brainly.in
Calculate the fundamental vibrational frequency HCl molecule, if the Vibrational Frequency Chemistry As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. Calculate the vibrational frequency of \(co\) given the following data: While vibrational frequencies are measured by spectroscopy, the normal modes of motion are inferred through theory because their visualization would require microscopy with. Vibrational frequencies have been used for many years by chemists to identify. Vibrational Frequency Chemistry.
From physicsopenlab.org
Water Molecule Vibrations with Raman Spectroscopy PhysicsOpenLab Vibrational Frequency Chemistry While vibrational frequencies are measured by spectroscopy, the normal modes of motion are inferred through theory because their visualization would require microscopy with. These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86.. Vibrational Frequency Chemistry.
From sites.cns.utexas.edu
Normal Modes of Vibration CH 431 Chemistry Vibrational Frequency Chemistry In the morse potential function, \(d_e\) is the bond dissociation energy, \(r_e\) is the equilibrium bond length, and \(a\) is a constant that. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. While vibrational frequencies are. Vibrational Frequency Chemistry.
From chem.libretexts.org
13.5 Vibrational Overtones Chemistry LibreTexts Vibrational Frequency Chemistry These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. In the morse potential function, \(d_e\) is the bond dissociation energy, \(r_e\) is the equilibrium bond length, and \(a\) is a constant that. As we’ve. Vibrational Frequency Chemistry.
From www.researchgate.net
DFT calculations of vibrational frequencies (IR) of the pure MP (a) and Vibrational Frequency Chemistry As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. Calculate the vibrational frequency of \(co\) given the following data: Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. While. Vibrational Frequency Chemistry.
From achs-prod.acs.org
Ab initio Modeling of the Vibrational SumFrequency Generation Spectrum Vibrational Frequency Chemistry As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. While vibrational frequencies are measured by spectroscopy, the. Vibrational Frequency Chemistry.
From www.researchgate.net
Calculated IR vibrational frequencies of the complexes and their Vibrational Frequency Chemistry Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. Calculate the vibrational frequency of \(co\) given the following data: While. Vibrational Frequency Chemistry.
From openmopac.net
Vibrational quantities Quantum versus Classical description Vibrational Frequency Chemistry In the morse potential function, \(d_e\) is the bond dissociation energy, \(r_e\) is the equilibrium bond length, and \(a\) is a constant that. Vibrational frequencies have been used for many years by chemists to identify bonding arrangements in molecules. Calculate the vibrational frequency of \(co\) given the following data: As we’ve emphasized many times in this course, within the born. Vibrational Frequency Chemistry.
From socratic.org
How does infrared spectroscopy identify functional groups? Socratic Vibrational Frequency Chemistry These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. Calculate the vibrational frequency of \(co\) given the following data: While vibrational frequencies are measured by spectroscopy, the normal modes of motion are. Vibrational Frequency Chemistry.
From www.researchgate.net
Schematic representation of vibrational modes of the H 2 O 2 isolated Vibrational Frequency Chemistry These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. While vibrational frequencies are measured by spectroscopy, the normal modes of motion are inferred through theory because their visualization would require microscopy with. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86.. Vibrational Frequency Chemistry.
From www.youtube.com
Calculation of Vibrational Frequency Hooke's Law (1) YouTube Vibrational Frequency Chemistry As we’ve emphasized many times in this course, within the born oppenheimer approximation, the nuclei. Mass of c = 12.01 amu, mass of o = 16 amu, the force constant \(k = 1.86. These complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are illustrated below. While vibrational frequencies are measured by spectroscopy, the. Vibrational Frequency Chemistry.